
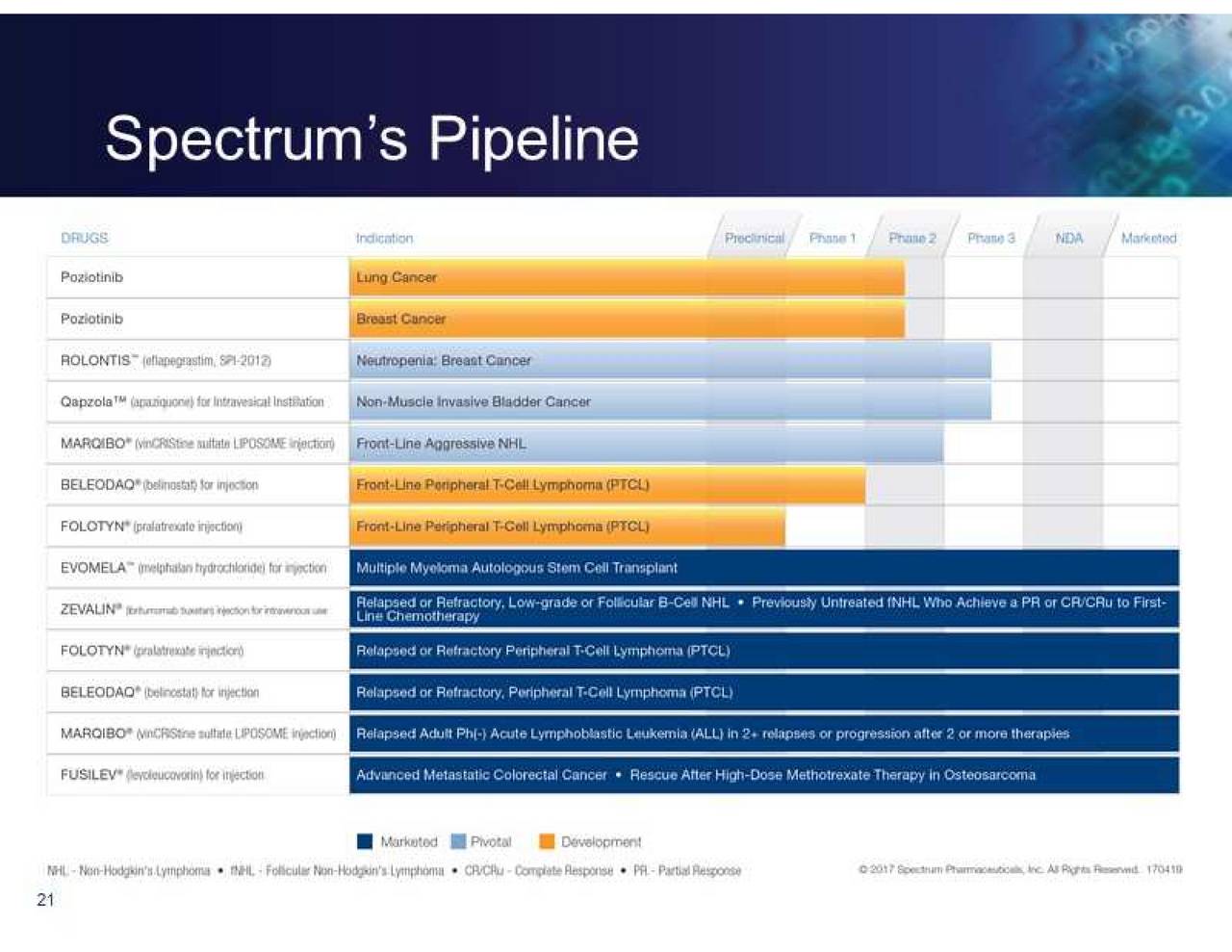
Spectrum launched ROLVEDON, which has an estimated market opportunity of approximately $2 billion, shortly following the FDA's approval. It is for adult patients with non-myeloid malignancies receiving myelosuppressive anti-cancer drugs associated with clinically significant incidence of febrile neutropenia. ROLVEDON was approved by the FDA in September 2022. The Company will focus efforts on driving growth for its recently launched commercial drug, ROLVEDON. Based on the anticipated cost savings from the restructuring, Spectrum believes it will be able to generate the working capital required to support its strategic refocusing through 2024. The Company will de-prioritize poziotinib program activities, effective immediately, and is in the process of reducing its R&D workforce by approximately 75%. We are grateful to the patients, families, and clinicians who participated in the poziotinib program and to the team members who have dedicated their time and efforts.” Riga continued, “We are committed to exploring potential strategic alternatives for poziotinib, including partnerships and business development opportunities, and will determine the best path forward in support of patients. “We continue to believe that poziotinib could present a meaningful treatment option for patients with this rare form of lung cancer, for whom other therapies have failed.” After multiple interactions with the FDA since ODAC, and following careful consideration, we have made the strategic decision to immediately de-prioritize the poziotinib program,” said Tom Riga, President and Chief Executive Officer of Spectrum Pharmaceuticals. “While we are not surprised by the CRL given the ODAC recommendation in September, we are disappointed. Based on the CRL, the Company would have to generate additional data including a randomized controlled study prior to approval. The FDA issued a CRL indicating the poziotinib application cannot be approved in its present form. Food and Drug Administration (FDA) regarding Spectrum’s New Drug Application (NDA) for poziotinib for the treatment of patients with previously treated locally advanced or metastatic non-small cell lung cancer (“NSCLC”) harboring HER2 exon 20 insertion mutations. (NasdaqGS: SPPI) (“Spectrum” or the “Company”), a biopharmaceutical company focused on novel and targeted oncology therapies, today announced that the Company has received a Complete Response Letter (CRL) from the U.S. Spectrum to explore strategic alternatives for the poziotinib program, including partnerships and business development opportunities. Immediately de-prioritizes poziotinib program, accelerates cost reductions, including 75% reduction in R&D related workforce.


 0 kommentar(er)
0 kommentar(er)
