
21, 2020 − I continue to learn new things! There is something called a double electron capture.
Electron capture formula full#
It is ejected from the nucleus where the electron reacts, so it is written on the right-hand side.Įxample #2: Here's another electron capture equation:Įxample #3: Write out the full electron capture equation for the following five nuclides.ġ8 38 Ar 38 80 Sr 53 125 I 69 168 Tm 99 247 EsĮxample #4: Here are five more to work on. This is the fingerprint of electron capture.)Ĥ) The atomic number goes DOWN by one and mass number remains unchanged.Įxample #1: Here is an example of a electron capture equation:Ģ) The electron must also be written on the left-hand side.ģ) A neutrino is involved. (Incidently, this cascade of electrons falling creates a characteristic cascade of lines, mostly (I think) in the X-ray portion of the spectrum.
Electron capture formula free#
One free electron, moving about in space, falls into the outermost empty level. These points present a simplified view of what electron capture is:ġ) An electron from the closest energy level falls into the nucleus, which causes a proton to become a neutron.Ģ) A neutrino is emitted from the nucleus.ģ) Another electron falls into the empty energy level and so on causing a cascade of electrons falling. In electron capture, something ENTERS the nucleus.
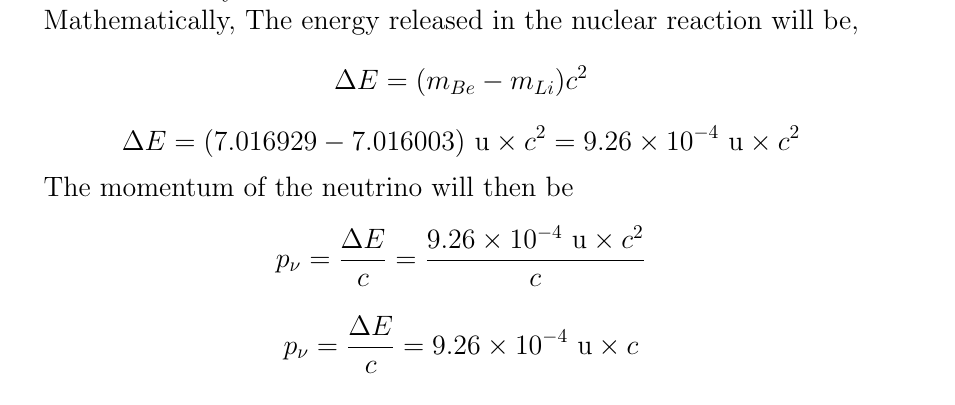
All other decays shoot something out of the nucleus. I'll only show the daughter nuclide:Ĩ 15 O 9 18 F 20 39 Ca 38 83 Sr 66 155 Dyīonus Example: Five more, but no answers.ġ1 21 Na 13 25 Al 16 30 S 21 42 Sc 29 60 CuĮlectron capture is not like the other three decays I have covered: alpha, beta, and position.

That's what I did with the answers.ħ 13 N 19 37 K 27 54 Co 30 61 Zn 31 68 GaĮxample #5: And here are five more. Sometimes, the teacher wants the neutrino left off the answer. Or, you might be using an older set of materials.Įxample #3: Write out the full positron decay equation for these five.Įxample #4: Here are five more to work on. You might wind up with an older teacher who insists on the older style of writing the neutrino. I couldn't make the formatting work, so I have to describe it in words. Notice that all the atomic numbers on both sides ADD UP TO THE SAME VALUE and the same for the mass numbers.īy the way, an older style for the neutrino symbol adds on two zeros where the atomic number and the mass number are placed, as well as dropping the subscripted e.
Some points to be made about the equation:ġ) The nuclide that decays is the one on the left-hand side of the equation.Ģ) The order of the nuclides on the right-hand side can be in any order.ģ) The way it is written above is the usual way.Ĥ) The mass number and atomic number of the neutrino are zero.ĥ) The neutrino symbol is the Greek letter "nu."Įxample #2: Here is another example of a positron decay equation: These points present a simplified view of what positron decay actually is:ġ) Something inside the nucleus of an atom breaks down, which causes a proton to become a neutron.Ģ) It emits a positron and a neutrino which go zooming off into space.ģ) The atomic number goes DOWN by one and mass number remains unchanged. Positron decay is like a mirror image of beta decay. ChemTeam: Writing Positron and Electron Capture Equations Writing Positron Decay and Electron Capture Equations Alpha Decay & Beta Decay Neutron Emission and Capture Gamma Decay Proton Emission and Capture Spontaneous Fission Radioactivity MenuĪ Brief Tutorial About Writing Nuclear Symbols


 0 kommentar(er)
0 kommentar(er)
